Specimen Collection and Transport Kit
BioSci provides three types of specimen collection and transport kits. The kit is intended for the collection and transport of clinical specimens (virus, chlamydiae, mycoplasma, or ureaplasma) from the collection site to the testing laboratory. The kit can be processed using standard clinical laboratory operating procedures for culture or subsequent testing. All kits are with US 510(k) registration or Europe MDR registration.
Virus Transport Medium Kit (VTM)

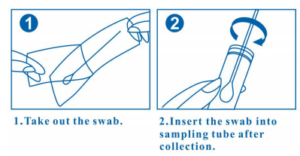
BioSci™ Virus Transport Medium Kit (VTM) is for collection and transport of viruses or chlamydiae actively. It contains a screw-cap tube pre-filled with 3ml medium to preserve the collected pathogen. It also contains one of or both nasal/oral swab(s). Performance of the VTM is evaluated by culture-based recovery studies for viruses and chlamydiae. The medium is non-toxic to host cells, and the infectivity of the pathogens in specimens remains after 48 hours of storage. The kit is with US 510(k) clearance and Europe MDR.
Inactivated Transport Medium Kit (ITM)
BioSci™ Inactivated Transport Medium Kit (ITM) is intended for the collection, inactivation, stabilization, and transportation of an unprocessed upper respiratory clinical specimen suspected of containing influenza A virus RNA from the collection site to the testing laboratory. The specimen collected in BioSci™ ITM is suitable for use with compatible molecular assays. The kit contains a detergent and a protein denaturant to inactivate influenza A, lyse cells, disrupt lipid membranes, denature proteins and enzymes, and preserve and stabilize influenza A virus RNA. It contains a screw-cap tube pre-filled with inactive medium, and/or one of nasal/oral/Mid-turbinate swabs. It contains guanidine hydrochloride. The kit is with US 510(k) registration or Europe MDR registration.
OmniTrans Transport System Kit (OTS)

BioSci™ OmniTrans Transport System Kit (OTS) is intended for use in the collection of clinical specimens (eg. sputum, nasopharyngeal/oropharyngeal swab, whole blood, urine, skin lesion material or exudate) potentially containing viruses, chlamydiae, mycoplasma, or ureaplasma and in their transport from the collection site to the testing laboratory. OTS is the first orthopoxvirus supported transport medium to receive US 510(k) clearance for use with samples of potentially containing monkeypox virus. It demonstrated the recovery of tested viruses, chlamydiae, mycoplasma, and ureaplasma at an acceptable rate when the specimen is stored at 2–25ºC for up to 48 hours. It contains one screw-cap tube containing specified volume of Transport Medium, for non-propagating transportation, and one regular flocking swab with 3 cm breaking point.The kit is with US 510(k) registration.